Elevating global standards for
Stem Cells
and Exosomes

Our breakthrough technology has cracked the elusive code of large scale stem cell and exosome manufacturing without compromising on quality. This enables us to maximize patient outcomes cost effectively.
Reimagine
Medicine
Stem cells are one of the most significant advancements in modern medicine, as they possess an unique ability to regenerate damaged tissues. This remarkable capability allows stem cells to address a variety of serious medical conditions that were previously considered untreatable.
Pioneering combination therapies using stem cells and exosomes
Both stem cells and exosomes possess unique, mutually exclusive tissue regenerative properties. We are the first large-scale manufacturer globally to combine both stem cells and exosomes into a single product, amplifying their reparative potential to deliver maximum therapeutic benefits for complex medical conditions.
Our core philosophy, "quality is the DNA of our products"
- Limited population doublings
- Xeno free cells (no animal products)
- CDSCO approved GMP manufacturing
- Extensive validation of our stem cells and exosomes
- Extensive validation for purity, potency, and sterility
First Trial in the World to Combine Stem Cells & Exosomes
Animal trials are a crucial step in evaluating the safety and efficacy of novel medical products. In a rat model of severe liver failure, our combined stem cell & exosome therapy successfully repaired liver damage—an effect absent in the control group. While 40% of untreated subjects succumbed, 100% of those treated survived, highlighting both the regenerative and life-saving potential of our product.
This achievement was accepted as a poster presentation at the American Association for the Study of Liver Diseases conference (Nov 2024), with human trials set to begin in Q1 2025.
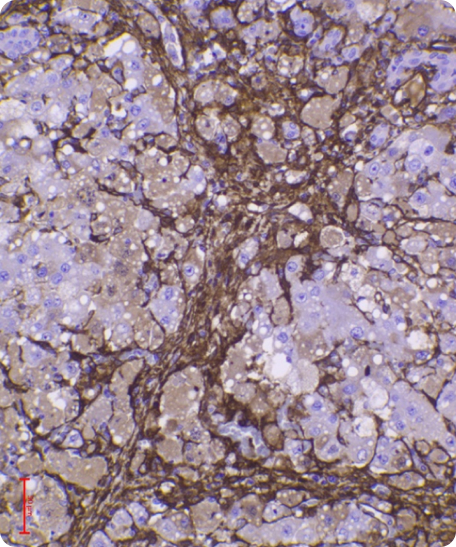
Liver damage with fibrosis
Before
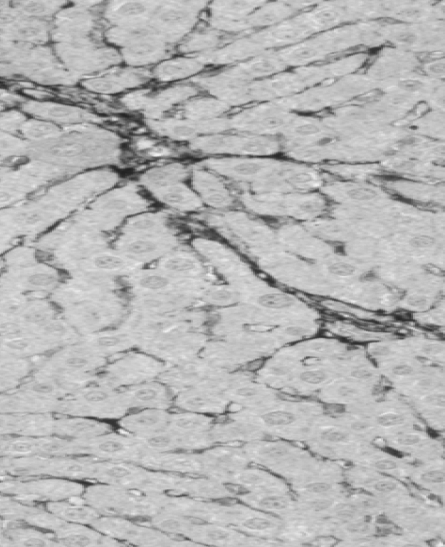
Liver damage with fibrosis
After
Treatable
Conditions
Extensive preclinical and early clinical trials demonstrate that stem cells have the potential to alleviate a wide range of challenging medical conditions including but no limited to:
Affordability
Is Our Core Value
Tulsi is challenging the widely accepted belief that you get what you pay for.' We have relentlessly pursued cost reduction without sacrificing quality, a core mission that reflects our founders’ commitment to democratizing this life-changing therapy. Our goal is to make this therapy accessible to millions. This achievement is the result of meticulous cost management during process development, a dedication to science over bloated management hierarchy, and strategic public-private partnerships.

One of the Only Physician-Led-Stem Cell and Exosome Manufacturing Companies

Dr. Sairam Atluri is one of the few physicians in the U.S. to be certified in Regenerative Medicine. His dual expertise as both a physician and a manufacturer uniquely positions him to optimize the production of stem cells and exosomes for superior tissue regeneration and disease reversal.
Dr. Sairam Atluri
(Founder and CEO)
Pioneering Collaboration
Through Partnerships
Indiana University
Our first trial targets patients with severe Liver Failure, a group with a high mortality rate and limited treatment options. Our Liver Failure trial is being conducted under the auspices of Dr. Naga Chalasani from Indiana University, one of the top three hepatologists in the world. Dr. Chalasani, along with his colleague Dr. Marwan, will play a pivotal role in the design, execution, and interpretation of this trial. Tulsi is also planning additional clinical trials in various other medical ailments.

Prestigious Hospitals
In line with our commitment to manufacturing high-caliber stem cells and exosomes, Tulsi will be launching multi-center, high-quality clinical trials at several prestigious medical institutes across India, including PGI Chandigarh and AIIMS Delhi. These trials are designed to rigorously assess both the safety and efficacy of our products prior to widespread therapeutic applications.

Nizam’s Institute of Medical Sciences (NIMS)
Nizam’s Institute of Medical Sciences (NIMS) is a renowned multispecialty center of excellence, offering medical care, education, and research facilities. Recognized as one of India’s premier government-funded medical institutes, NIMS stands at the forefront of healthcare innovation. Tulsi is proud and honored to collaborate with NIMS to manufacture premier-quality stem cells and exosomes and conduct clinical trials. This public-private partnership is unprecedented on a global scale.

Got Questions?
We're Here to Assist You!
6-3-661/7, Sangeet Nagar, Somajiguda, VTC, Hyderabad, Telangana, 500082
Dr. Sairam Atluri
(Founder and CEO)
- Published the first-ever prospective controlled trial using stem cells to treat severe chronic low back pain, which also stands as the largest trial involving non-cultured bone marrow stem cells for this condition.
- Was the first in the US to publish an article about treating severe covid -19 patients with Stem Cells
- His pioneering technique for bone marrow aspiration extracted the highest number of bone marrow stem cells in the published literature.
- As a co-editor of two regenerative medicine textbooks and a contributor to chapters in other medical texts, Dr. Atluri has shared his expertise widely.
- Dr.Atluri is a frequent speaker at all the top Regenerative Meetings in the US and India.
- Part of the team that developed regenerative treatment guidelines for low back pain for the American Society of Interventional Pain Physicians (ASIPP).